ChoMaster®EPO cells
ChoMaster®EPO is a genetically engineered CHO cell line secreting human erythropoietin (EPO) in chemically defined protein- and peptide-free minimal culture media developed and manufactured by Cell Culture Technologies.
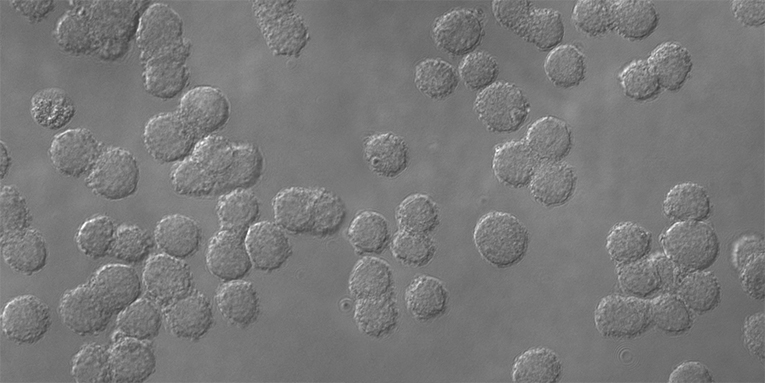
Morphology of ChoMaster®EPO cells throughout cultivation in protein- and peptide-free conditions on medium ChoMaster® HTS. Courtesy of the Cell Physiology and Cellular Engineering group, ZHAW Waedenswil, Switzerland
ChoMaster®EPO is provided as open-source technology package featuring culture media and simple working protocols to get acquainted with the minimal maintenance technique.
ChoMaster®EPO is part of Cell Culture Technologies’ ChoMaster® Biosimilar Series.
Cell line
CHO-K1 derived ChoMaster®EPO cells were transfected with the synthetic plasmid pJ603:115122 driving codon-optimised human EPO expression under CMV-control after adaptation to chemically defined, protein- and peptide-free ChoMaster® and FMX-8 minimal culture media developed by Cell Culture Technologies.
- Routine maintenance
Routine maintenance of ChoMaster®EPO cells in static or agitated culture systems does not require any supplementation of proteins, peptides, hydrolysates or complex additives to the above culture media.
- Growth kinetics
The growth kinetics of ChoMaster®EPO cells is characterised by a doubling time of 20h in the exponential growth phase of agitated cultures, while cell densities range from the single cell clone to million cells per ml.
- Volumetric productivity
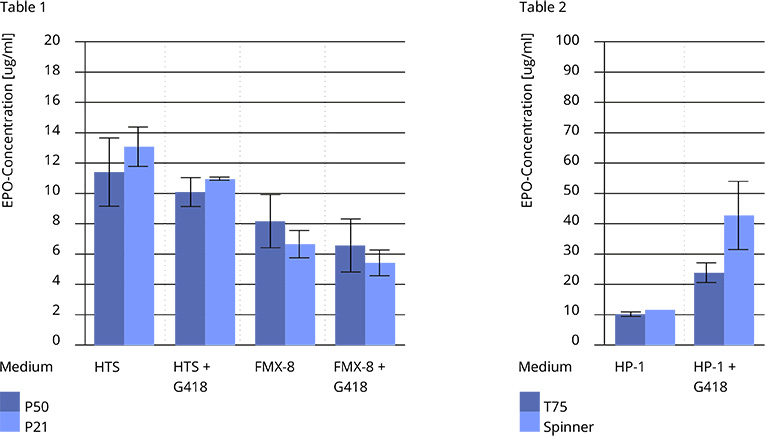
The volumetric productivity of ChoMaster®EPO cells strictly depends on the nutrient strength of the minimal culture media. While ChoMaster®EPO cells typically produce up to 8 mg/l rh-EPO in FMX-8 medium under static culture conditions (Tab. 1), a simple increase of amino acid and glucose concentrations results in a dramatic increase of the EPO concentration up to 50 mg/l under agitated culture conditions (ChoMaster® HP-1, spinner flasks, Tab. 2). Stability of glycoprotein secretion was proved for over 30 passages (Tab. 1).
We suggest adding G418 to the culture medium routinely in order to maintain high levels of recombinant protein secretion.
Routine maintenance of ChoMaster®EPO cells in static or agitated culture systems does not require any supplementation of proteins, peptides, hydrolysates or complex additives to the above culture media.
The growth kinetics of ChoMaster®EPO cells is characterised by a doubling time of 20h in the exponential growth phase of agitated cultures, while cell densities range from the single cell clone to million cells per ml.
The volumetric productivity of ChoMaster®EPO cells strictly depends on the nutrient strength of the minimal culture media. While ChoMaster®EPO cells typically produce up to 8 mg/l rh-EPO in FMX-8 medium under static culture conditions (Tab. 1), a simple increase of amino acid and glucose concentrations results in a dramatic increase of the EPO concentration up to 50 mg/l under agitated culture conditions (ChoMaster® HP-1, spinner flasks, Tab. 2). Stability of glycoprotein secretion was proved for over 30 passages (Tab. 1).
We suggest adding G418 to the culture medium routinely in order to maintain high levels of recombinant protein secretion.
The 22-years-old history of the host cell line used to generate ChoMaster®EPO cells has been recorded since the very first days of cultivation in Cell Culture Technologies’ minimal media. A wide range of recombinant proteins have been successfully expressed with our CHO-K1 cells adapted to our minimal nutrient mixtures since 1993.
Several academic institutions, small biotechs and big pharma companies licensed our unique know-how to implement and develop tailor-made bioprocess solutions. ChoMaster®EPO cells were genetically engineered by Jack Rohrer, Bettina Keller Abu Seda and Jenny Pally-Eggenschwiler, Institute of Cell Biology, Zurich University of Applied Sciences, Waedenswil, Switzerland.
Culture media
ChoMaster® HTS, ChoMaster® HP-1 and ChoMaster® HP-5 media; FMX-8 medium
ChoMaster® minimal media are exclusively made of small molecules of highest purity characterised by their respective CAS/EINECS numbers. ChoMaster® HP media are fortified nutrient mixtures that contain no ingredients of human or animal origin, no recombinant proteins, peptides, peptones or yeast extracts, and no hydrolysates. A proprietary manufacturing technique coupled with the detailed estimate of the nutritional requirements of ChoMaster®EPO cells have led to the development of several ChoMaster® media formulations to fit several possible culture processes, from single cell cloning (ChoMaster® HTS) over routine maintenance (ChoMaster® HP-1) to high density perfusion culture (ChoMaster® HP-5).
FMX-8 medium, Ferruccio Messi’s medium X version no. 8, is a chemically defined minimal nutrient mixture for CHO cells made of 43 small molecules. The complete formulation of the FMX-8 medium with the identity and the concentration of every ingredient was published in 1991 and 1993 by Ferruccio Messi, and 1995 by Michael Zang, Helmut Trautmann, Christine Gandor, Ferruccio Messi, Fred Asselbergs, Christian Leist, Armin Fiechter and Jakob Reiser.
ChoMaster®EPO cells easily proliferate in the protein- and peptide-free ChoMaster® and FMX-8 minimal culture media developed and manufactured by Cell Culture Technologies. While full reproducibility of the media composition allows wide control over cell proliferation and recombinant protein expression, ChoMaster and FMX-8 media can be obtained under a toolbox format to investigate the characteristics of the secreted glycoprotein of interest in great details. Our Media Toolbox Kits consist of a basal medium combined with a set of seven concentrated solutions for investigational purposes. For example, the Titer Enhancer Toolbox consists of a glucose-free basal medium – either ChoMaster or FMX-8 – and seven concentrated solutions with amino acids formulated into metabolically related groups for the purpose of increasing cellular productivity. When properly combined, the components of the Media Toolbox Kits may generate new glycoproteins (isoforms) of interest.
Please find a selection of available ChoMaster & FMX-8 Media Toolbox Kits below:
Methods
ChoMaster®EPO erases words such as « animal derived components » or « complex supplements » from R&D paperwork relating to recombinant human EPO expression. In fact, absolutely no use of components of animal origin or chemically undefined extracts is required for producing EPO with ChoMaster®EPO cells. The total absence of complex ingredients from our minimal nutrient mixtures increases the degree of reproducibility of the culture process while reducing the risk of contamination to a minimum and facilitating downstream procedures.
Technical support regarding freezing, thawing and maintaining ChoMaster®EPO cells in ChoMaster® and FMX-8 media is provided together with the media and the cells, so that recombinant EPO expression can be immediately accomplished.
Ordering information & Terms of use
ChoMaster®EPO cells are deposited and certified by the Culture Collection of Switzerland, Waedenswil, Switzerland, and may be solely obtained from Cell Culture Technologies by signing a Material Transfer Agreement (MTA) in advance. Such MTA may be obtained by contacting Cell Culture Technologies directly.
ChoMaster® is a registered trademarks of Dr. F. Messi Cell Culture Technologies.